A Tik Tok video claims that COVID-19 swabs sterilized with ethylene oxide gas are killing people. However, US and Australian public health agencies say the chemical is safely used to sterilize a range of medical products, the CDC says the test kits are not dangerous, and a professor said there is no basis for claims that the tests can lead to cancer.
Also Read: Pilots Vaccinated Against COVID-19 Are Not Barred From Flying In Canada
"That's gonna kill us, that's actually killing people," claims a man in a video posted to Tik Tok on July 17, 2021. In it, he refuses to take a COVID-19 test because the package of the testing swab shows it has been sterilized using ethylene oxide (EtO or EO), which he links to cancer and the altering of human DNA. Text across the video says: "Covid testing kit in Australia."
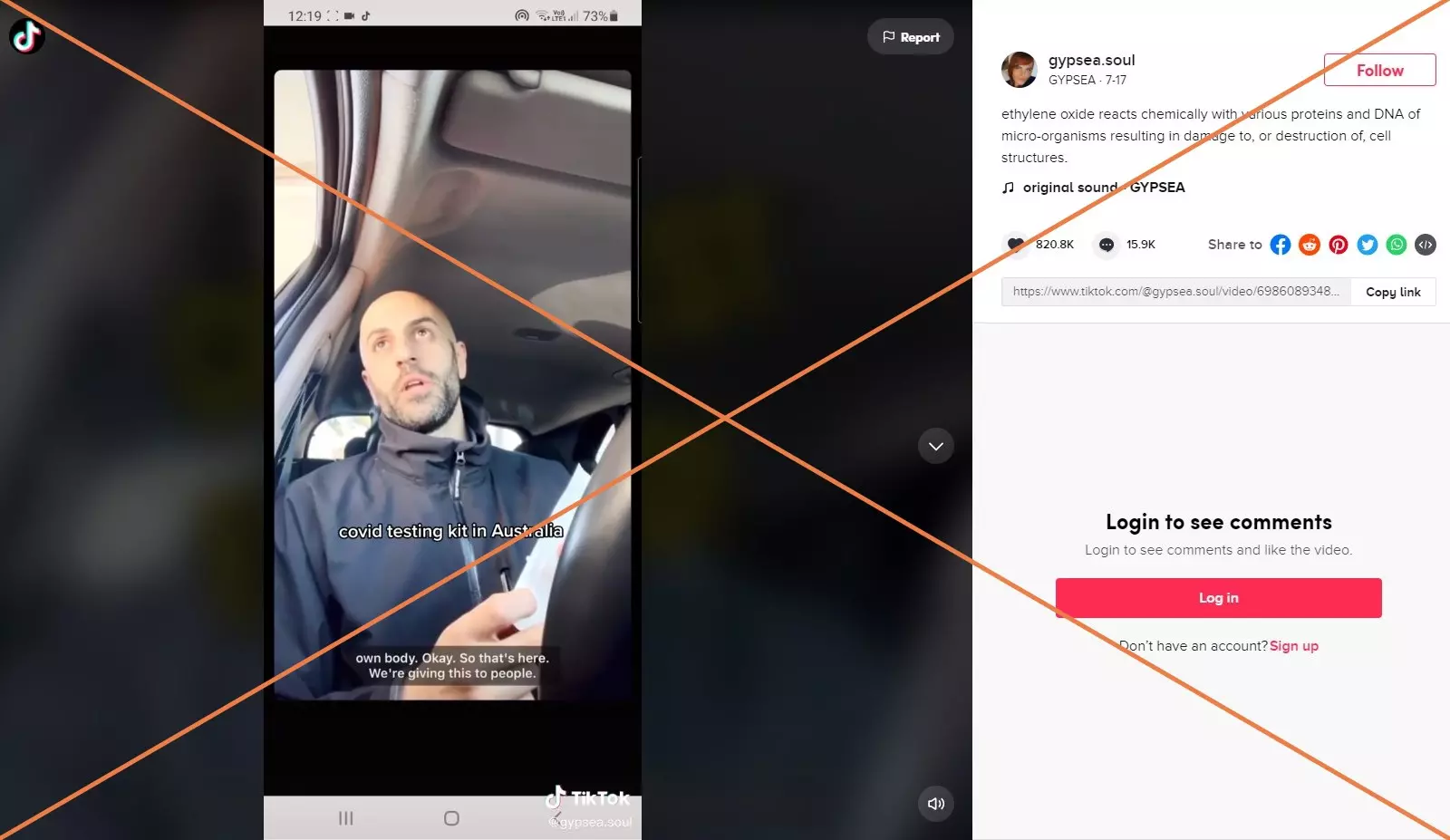 Screenshot of a Tik Tok video taken July 26, 2021
Screenshot of a Tik Tok video taken July 26, 2021 The video which has been viewed more than 8.7 million times and has received more than 820,000 likes on Tik Tok, was shared outside of Australia on Facebook here, and here and on Instagram here.
It spread as the highly contagious coronavirus Delta variant pushed the number of cases in the United States higher and Australia's Victoria state entered its fifth lockdown, with misinformation about COVID-19 continuing to circulate.
Both Australia and the US use ethylene oxide to sterilize medical supplies.
The man in the video correctly states that ethylene oxide is used in antifreeze, that it is carcinogenic and that it can alter DNA, our genetic information.
But its use in medical devices is subject to safety guidelines and the majority of COVID-19 test swabs are, in fact, not sterilized, health authorities say.
Jade Fulce, a Centers for Disease Control and Prevention (CDC) spokeswoman, said that COVID-19 test kits are not a cause for concern.
She told AFP on July 22, 2021: "COVID-19 tests and associated clinical specimen collection devices are not dangerous when used in accordance with the manufacturer's instructions."
Also Read: Joe Biden Falsely Claims Vaccinated People Cannot Contract COVID-19
Sarah Racic, a spokeswoman from the Australian Department of Health, told AFP on July 23, 2021 that there is a "lack of evidence to suggest that residual ethylene oxide exposure from the transient use of ethylene oxide sterilized COVID-19 PCR test swabs causes cancer or DNA mutations."
She also said that "the majority of swabs used for collection of COVID-19 samples are non-sterile and therefore are not exposed to ethylene oxide."
For those that are sterilized, the process works because the gaseous EO does not stick to the swabs, Annette Beck-Sickinger, professor of biochemistry and bioorganic chemistry at the University of Leipzig, said for a previous fact check in German.
"All the stories that testing triggers 'cancer' are really unfounded," she said.
"If you have treated the test sticks with EO and then packaged it sterilely, then the packaging is usually permeable to the gas," Beck-Sickinger explained. This means that manufacturers can easily pump out the gas, but bacteria can no longer get into the packaging.
The US National Cancer Institute confirms on its website that ethylene oxide can damage DNA, which is what makes it an effective sterilizing agent and also accounts for its link to cancers.
But adverse health effects associated with ethylene oxide exposure are highly unlikely for those outside of specialized workplaces that use the gas, says Food Standards Australia New Zealand, a government health agency.
Even though there are "potential human health risks," the US Environmental Protection Agency says it considers EtO "critical for sterilizing medical equipment and necessary to protect public health." It adds that many herbs and spices are also treated with EtO to reduce bacterial levels.
The substance has "excellent microbicidal activity" but, because EtO is absorbed by many materials, the CDC website says that "following sterilization the item must undergo aeration to remove residual EtO." It sets guidelines for allowable EtO limits "that depend on how the device is used, how often, and how long in order to pose a minimal risk to patients in normal product use."
The US Food and Drug Administration, the industry regulator, says that for many medical devices, EtO sterilization "may be the only method that effectively sterilizes and does not damage the device during the sterilization process."
A spokesperson for the FDA told AFP on July 22, 2021 that ethylene oxide is "considered a safe and effective method that helps ensure the safety of medical devices and helps deliver quality patient care."
Before sterile medical devices go on the market, the FDA also makes sure that sterilization methods are "in accordance with internationally agreed upon voluntary consensus standards," the spokesperson said, adding these determine "the acceptable levels of residual ethylene oxide and ethylene chlorohydrin left on a device."
"These standards help ensure levels of ethylene oxide on medical devices are within safe limits since long-term and occupational exposure to ethylene oxide has been linked to cancer," the FDA website says.
Also Read: Pfizer-BioNTech COVID-19 Vaccine Does Not Contain Graphene Oxide
Standards are also strictly followed in Australia according to Racic, the Australian Department of Health spokeswoman, who said manufacturers are required to perform studies that "ensure the final product is sterile but also safe to use, including any residues."
AFP Fact Check has debunked several false claims about COVID-19 test kits here.
(Except for the headline, this story has not been edited by BOOM staff and is published from a syndicated feed.)